ALZHEIMER’S RESEARCH STUDIES EFFECTS OF BLOOD PLASMA TREATMENTS
Texas Health Dallas is one of three national sites conducting a unique clinical trial testing the theory that blood plasma from young, healthy people can be injected into older adults to treat Alzheimer’s disease.
“The current drugs we have only mask the symptoms of Alzheimer’s while the disease continues to progress,” said Dr. Diana Kerwin, a geriatric medicine specialist at Texas Health Dallas and lead investigator of the study, in a release. “Finding a treatment for Alzheimer’s is important to people afflicted by it and their families, but it has far-reaching implications as the baby boomer generation ages and more people are living longer. It’s a national and worldwide concern.”
“The current drugs we have only mask the symptoms of Alzheimer’s while the disease continues to progress.”
Dr. Diana Kerwin
Stanford University and University of California, San Francisco also are conducting similar studies, according to Texas Health Resources.
Each study is independently reviewed and funded. The Texas Health Dallas study is being funded by the Josephine Hughes Sterling Foundation.
The studies will help give a better understanding of whether human plasma — the straw-colored liquid component of blood that comprises a little more than half of the body’s blood volume — has the ability to repair cognitive function in older adults with Alzheimer’s, the release said.
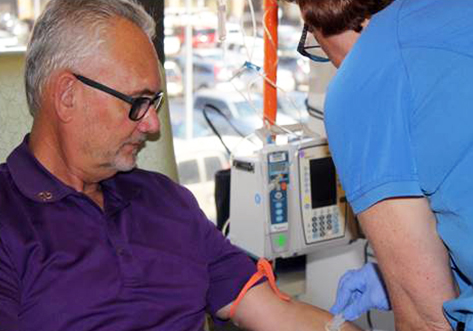
Jeff Rushing is involved in an Alzheimer’s study at Texas Health Dallas. [Photo courtesy of Texas Health Resources]
Previous studies looked at how the brains in older mice improved after receiving plasma from younger ones, while other investigations determined that plasma from human umbilical cords and young adults improved cognitive function in older mice.
One of the participants in the study is Jeff Rushing, an Alzheimer’s patient who is still able to enjoying watching westerns on television, and who volunteers at his local church. Fellow volunteers keep a watchful eye on Rushing.
“The guys know I have Alzheimer’s,” Rushing said in the release. “So they watch after me because a lot of times, I don’t know where I should be going or what I need to do on certain projects.”
Rushing’s wife, Julie, said the research gives the couple hope, even though the results of the investigation aren’t known and Rushing, 62, hasn’t shown signs of significant improvement yet. He’s one of more than 5 million Americans who have Alzheimer’s, and estimates by the Alzheimer’s Association are that by 2050, the number of affected individuals could rise to 16 million.
Texas Health Dallas is a part of Arlington-based Texas Health Resources, one of the largest faith-based, nonprofit health systems in the nation.
DO LOUD HEADPHONES, CONCERTS CAUSE HEARING LOSS?
Your parents probably told you that listening to loud music through headphones or going to a rock concert was going to damage your hearing.

Colleen Le Prell
Researchers have hypothesized about recreational noise exposure — so-called “hidden hearing loss” — for years, but the damage is difficult to detect using conventional hearing tests.
A recent study by researchers at the Callier Center for Communication Disorders at the University of Texas at Dallas doesn’t support the idea that headphones or concerts damage your hearing.
The author of the paper published in Frontiers in Neuroscience is Colleen Le Prell, head of the doctor of audiology program at UT Dallas.
Her study’s participants attended a “loud” event of their choosing — concerts, clubs, or music festivals — and recorded the amount of time spent there and how it was.
Researchers conducted pre- and post-exposure tests to measure any changes in the participants hearing and speech abilities.
What they found was no reliable hearing loss, but the tests did show noise-induced changes in the processing of speech in a noisy background, according to UTD.
“In these tests, a recorded speaker tells the subject to say a certain word,” Le Prell said in the release. “As the level of background noise and the level of speech come closer together, the task gets harder. We found that the day after the noise exposure, the more noise exposure they had experienced, the poorer they did on the test. They missed more words.”
Those changes were temporary, and there was no evidence of either a temporary or a permanent change in neural function or the function of sensory hair cells in the inner ear, the release said.
UTA PROFS GET NSF GRANT TO STUDY HURRICANE DEBRIS
Major hurricanes leave behind paths of destruction, and two professors at the University of Texas at Arlington have received funding from the National Science Foundation to study waste debris left behind by Hurricane Harvey on the Texas Gulf Coast.

Anand Puppala

Junzhou Huang
Anand Puppala, associate dean of research in UT Arlington’s College of Engineering, will be principal investigator on the study and Junzhou Huang, associate professor in the Computer Science and Engineering Department, will be co-principal investigator in the project that seeks to quantify the debris by using photogrammetry from smart phones and unmanned aerial vehicles.
Photogrammetry is the use of photography in mapping and surveying to measure the distances between objects.
The UTA team received a grant for $34,137 as part of $5.3 million in 59 grants focused at studying the effects of recent hurricanes Harvey, Irma, and Maria.
The grants are aimed at helping scientists better understand how such major disasters happen and how to best respond, according to the NSF.
NANOLOGY ENROLLS FIRST PATIENT IN PHASE 2 TRIAL
NanOlogy LLC, the Fort Worth-based clinical-stage pharmaceutical company, has enrolled its first patient in a Phase 2 clinical trial of its ovarian cancer treatment NanoPac.
The intraperitoneally administered nanoparticle paclitaxel sterile suspension is part of a nanoparticle technology platform developed by NanOlogy.
“Systemically administered paclitaxel has been shown to be effective in ovarian cancer, but is limited by its adverse effects,” Gere diZerega, vice president of medical affairs at NanOlogy, said in a release. “We are attempting to show that a single, IP-instilled dose of NanoPac will effectively treat the cancer with high locally sustained concentrations of the drug and no contribution to systemic adverse effects.”
NanOlogy said it has an extensive clinical development program underway for the NanoPac sterile suspension, including clinical trials in ovarian cancer (with orphan drug designation), prostate cancer, pancreatic cancer, and pancreatic mucinous cysts.
You can read more about the NanOlogy trial in the Fort Worth Business Press here.
Get on the list.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.
And, you’ll be the first to get the digital edition of our new Dallas Innovates magazine:
The annual edition publishes in January.