Dallas-based Lupagen Inc., a pioneer in cell and gene therapy delivery, has announced a new development and supply agreement with Fresenius Kabi, a global leader in injectable medicines, infusion technology, and clinical nutrition.
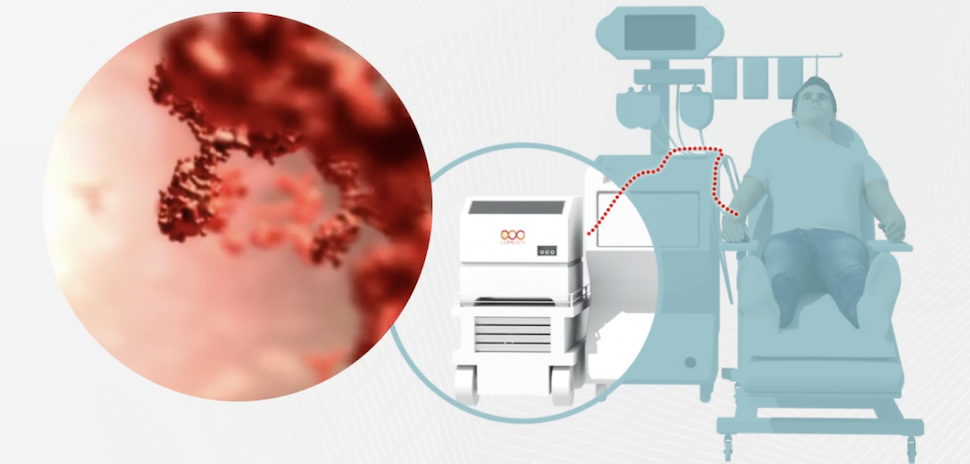
The deal aims to bring innovative cell and gene treatments closer to patients by advancing Lupagen’s proprietary cell and gene therapy delivery system, according to a news release. Lupagen’s system focuses on simplifying and reducing costs for cell and gene therapy through a bedside procedure.
Under the agreement, Fresenius Kabi will provide expertise from its transfusion medicine unit to optimize Lupagen’s platform for delivering cell and gene therapy treatments at a patient’s bedside.
With a U.S. headquarters in Illinois and global headquarters in Germany, Fresenius Kabi has an international presence.
Lupagen Co-founder and CEO Nipon Das says the collaboration with the global leader in blood and cell technologies “accelerates deployment of our solution to patients who need these treatments the most.”

Nipon Das, CEO of Lupagen. [Photo: Lupagen]
Das says the biotech wants to make cell and gene therapy as common and accessible as standard blood transfusion or dialysis procedures.
Founded in 2017, the biotech is headquartered in Dallas at Cypress Waters with a development site in Chicago. Lupagen is developing technologies to deliver cell and gene therapies, including CAR-T cancer treatments, gene editing, and immunotherapies. The therapies are created using a patient’s own cells processed outside the body.
“The biggest challenges that the cell and gene therapy community are facing are cost and complexity of production,” Das told Dallas Innovates last year. The company is tackling both.
Working internally and in partnership with other biopharma companies, Lupagen also develops nanoparticle-based gene therapies that can be delivered by its point-of-care system.
Collaborating for cell and gene therapy bedside access
Cell and gene therapies have shown promise for many conditions, including cancers and hematologic, autoimmune, and rare diseases.
Together, Lupagen and Fresenius Kabi aim to overcome key barriers around manufacturing, logistics, and costs that have limited patient access to those therapies. Their “to the bedside” approach could help open cutting-edge treatments to local hospitals and patients worldwide.
“Cell and gene therapies have helped treat or cure some of society’s most complex and challenging diseases, but more needs to be done to bring the delivery of these technologies closer to patients,” Fresenius Kabi’s Bryan Blickhan said in a statement.
Blickhan, the company’s senior vice president of R&D and device production, transfusion medicine, and cell therapies, called the agreement with Lupagen a “great step” toward more affordable and scalable therapies for people.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.