A team of UT Dallas bioengineers has developed a rapid test for viruses that delivers results as accurate as lab tests within 30 minutes—and they’ve launched a new company to commercialize it.
The test is 150 times more accurate than traditional rapid tests and matches the accuracy of polymerase chain reaction (PCR) tests, the team says.
Dr. Zhenpeng Qin, associate professor of mechanical engineering, mechanical engineering doctoral student Yaning Liu, and Dr. Haihang Ye, research associate in mechanical engineering, are part of the team that developed the method, called DIgitAl plasMONic nanobubble Detection (DIAMOND).
While the current study focused on respiratory syncytial virus (RSV), Dr. Qin says the tech can be applied to other viruses, such as those that cause COVID-19 and the flu. Researchers also plan to use the platform to identify cancer biomarkers.
‘We should be able to save lives’
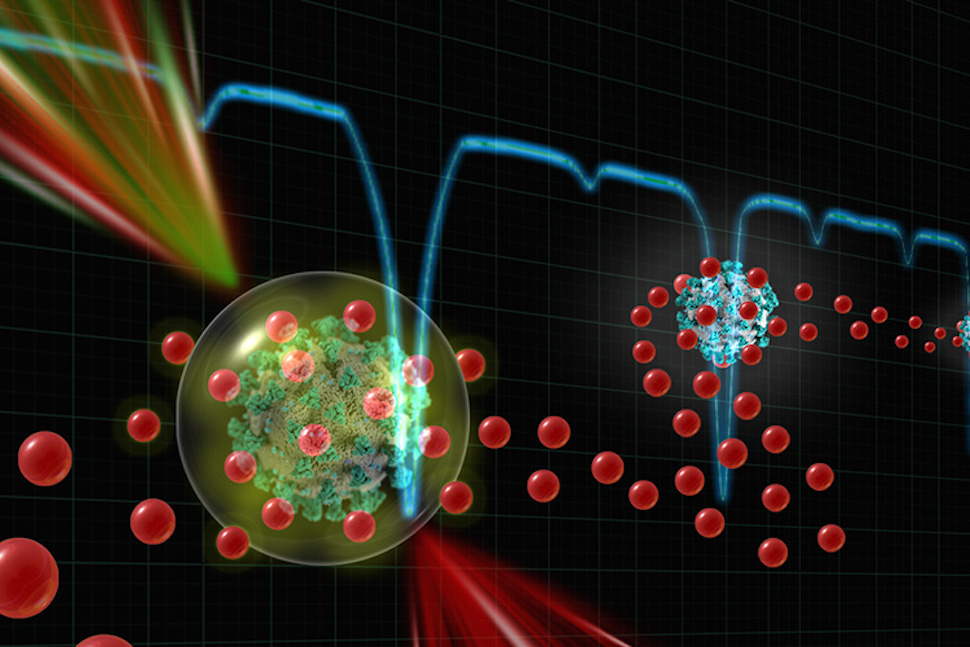
This illustration shows a patient sample, with virus-recognizing gold nanoparticles, moving through a laser checkpoint. The laser activates the nanoparticles on the sample, which absorbs the laser’s energy and expands and generates a vapor bubble. A large nanobubble signals the presence of a virus. [Image: UT Dallas]
DIAMOND works by attaching gold nanoparticles to antibodies against the virus being tested. These combination molecules are then mixed with a patient’s nasal swab sample. If the sample contains a virus, the antibodies labeled with gold nanoparticles will bind with proteins on the virus’s surface.
“If we can detect pathogens earlier, we should be able to save lives,” said Dr. Ye, a co-corresponding author of the study, in a statement. “Our detection is quick, accurate and sensitive.”
The team believes their new strategy paves the way for much faster results than PCR tests, which often take a day or more to reveal results—as countless millions of us know from the last two years of COVID-19 testing.
Key benefits of the tests include the fact that they’re “inexpensive and do not require complex liquid handling or extensive sample preparation.”
Like sending a bag through a security checkpoint
 Ye compared the test’s process to sending luggage down an airport’s security conveyer belt.
Ye compared the test’s process to sending luggage down an airport’s security conveyer belt.
“When bags go through the checkpoint, a scanner will image what’s inside of the bag, and if there’s anything suspicious, there’s an alert,” Ye said in the statement. “That’s what we’re doing with this technology. We use two lasers: one to scan the sample and one to tell us whether there’s a virus inside.”
The first laser activates the gold nanoparticles, which expand when they absorb the laser’s energy. If they expand strongly enough, the nanoparticle will boil the surrounding water, generating vapor bubbles. The size of the nanobubbles determines the test result—with large nanobubbles signaling presence of a virus.
Launching Avsana Labs to commercialize the tech
Qin published the team’s study online March 30 in Nature Communications. He and his team in the Erik Jonsson School of Engineering and Computer Science have launched a new company, Avsana Labs, to commercialize the tech through the university’s Venture Development Center.
The technology would require FDA approval before it could be publicly available, after which researchers would bring it to hospitals, labs, drive-thru pharmacies and—eventually—home tests.
Avsana and Dr. Qin won the grand prize at MassChallenge
While Avsana Labs works to commercialize the tech, researchers will continue to improve the platform and expand its applications, the team says.
Avsana and Qin, the company’s president, won the grand prize in the MassChallenge North Texas pitch competition in January, receiving $10,000 to help commercialize the tech.
Nearly $3 million in grants, plus collaborators’ assistance
To support the research behind the breakthrough, Qin received $2.5 million in grants over five years from the NIH’s National Institute of Allergy and Infectious Diseases, as well as a $293,000 grant from the Department of Defense’s Congressionally Directed Medical Research Programs.
Other UT Dallas contributors to the Nature Communications study included Chen Xie, mechanical engineering doctoral student, and Peiyuan Kang PhD ’20, who’s now a researcher at Harvard Medical School. The research included collaborators from UT Southwestern Medical Center: pediatric research scientist Dr. HoangDinh Huynh and Dr. Jeffrey S. Kahn, chief of pediatric infectious disease.
In addition, a team of UT Dallas engineering students contributed to the device through UTDesign Capstone, a Jonsson School engineering and computer science program.
UT Dallas has filed a provisional patent on the technology, and Qin and Kahn hold equity interest in Avsana Labs, the university said.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.





![Dr. Justin Lonon, vice chancellor of Dallas College, addresses the crowd at the recent Goldman Sachs 10,000 Small Businesses Dallas Graduation. [Photo: 10KSB]](https://s24806.pcdn.co/wp-content/uploads/2021/06/GoldmanSachs-10KSB-4992-970-970x464.jpg)

























