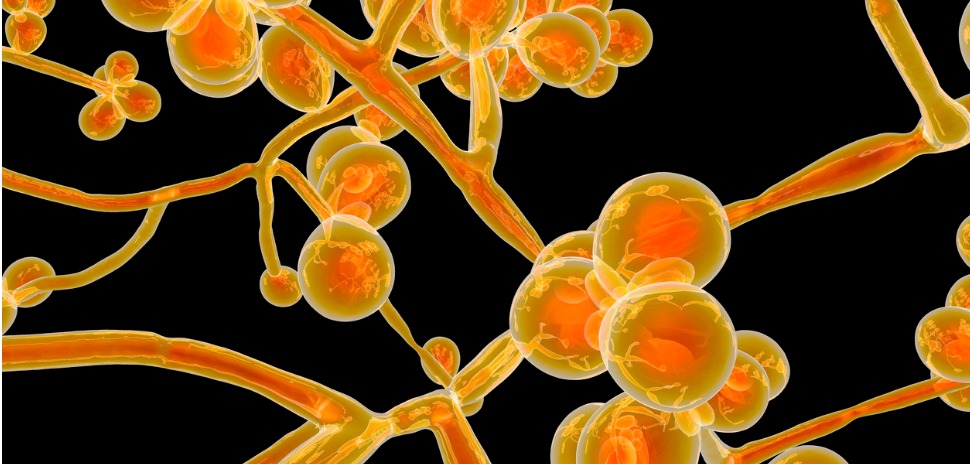
A deadly yeast that claims the lives of one in three victims has historically been difficult to diagnose and treat, but new molecular tests from MycoDART may change those odds.
Carrollton-based clinical molecular laboratory MycoDART’s new test has the potential of saving patients valuable hours and even days when it comes to properly treating the deadly yeast, and can even be run over environmental surfaces to test for the presence of Candida auris (C. auris).
C. auris is a type of yeast that can cause severe sickness in hospitalized patients. High risk patients include those with diabetes, recent surgeries, those who have spent time in nursing homes, and patients who have lines and tubes attached to their bodies.
C. auris can be cured, but is often resistant to anti fungal medications, and becomes more dangerous if given enough time to reach a patient’s bloodstream.
It can be caused from ear infections, wound infections, and bloodstream infections. It was first discovered in Japan in 2009, and is considered an ’emerging pathogen’, according to the CDC.
While it is possible to cure C. auris, between 30 and 60 percent of patients who have contracted the yeast have died from the infection, though many patients had prior illnesses that put them at a higher risk.
The infection is typically diagnosed by a blood culture, but can often be mistaken for a different type of Candida or different forms of yeast and consequentially mistreated. The culture can also take more than one day to show results, which can be deadly for patients since the likelihood of Sepsis and other related illnesses becoming fatal increases by 5 percent each day the infection goes undiagnosed.
MycoDART-PCR’s new test will have the ability to give doctors results in hours rather than days, saving patients vital time and getting them quicker treatment for their diagnosis. The molecular test will be able to correctly identify C. auris with a specificity above 95 percent.
“MycoDART’s senior medical and clinical staff recognized several years ago that yeast and mold infections were on the rise among cancer patients, transplant patients, and other immunosuppressed individuals,” CEO Dave Murcott said in a statement. “When we saw how seriously the CDC took C. auris, we put it straight into R&D so we could add it to our Candida panel as soon as possible,”
MycoDART, which was named one of Health Wildcatter’s six startup healthcare accelerators, works to combat exposure to environmental toxins by using patented testing and pre-clinical testing for researchers, in an effort to cure illnesses linked to environmental toxic mold exposure.
The laboratory is currently in the pre-launch stage for the new Dual Amplification Realtime Polymerase Chain Reaction DNA test, which will be able to accurately detect six different species of Candida.
The test will need FDA approval before it can be manufactured and distributed to hospitals and medical facilities, though tests can be ordered through a single licensed lab.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.