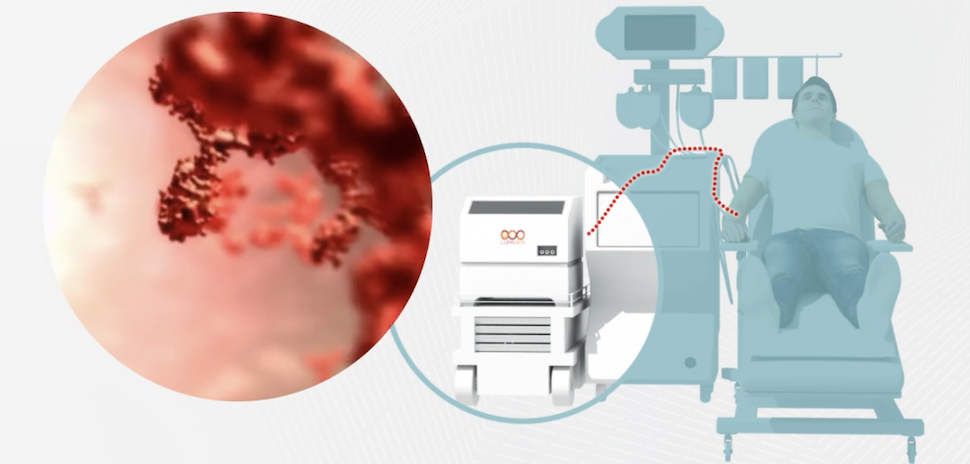
History was made Friday when Boston’s Vertex Pharmaceuticals and Switzerland’s CRISPR Therapeutics announced that the sickle cell disease drug CASGEVY is the first CRISPR-based gene-editing therapy to be approved in the U.S.
CASGEVY received FDA approval as a one-time intravenous suspension to treat sickle cell patients age 12 and older who have recurrent vaso-occlusive crises, or VOCs—when sickled red blood cells block blood flow, depriving tissues of oxygen.
The companies say around 16,000 patients may be eligible for this “durable” one-time therapy offering “the potential of a functional cure” for the disease, by eliminating severe VOCs and hospitalizations caused by them.
Medical City Children’s is one of only 9 U.S. hospitals on initial treatment center list
Administering CASGEVY requires specialized experience in stem cell transplantation. On Friday, the companies announced that only nine hospitals in the U.S. were part of its initial network of authorized treatment centers—and one of them is Medical City Children’s Hospital in Dallas.
Medical City Children’s is one of only two hospitals in Texas that had been activated Friday in the CASGEVY authorized treatment center network. The other is Methodist Children’s Hospital in San Antonio.
The seven other authorized centers across the U.S. include hospitals and cancer centers in Boston, Washington D.C., Los Angeles, Chicago, Nashville, and Columbus, Ohio.
“CASGEVY’s approval by the FDA is momentous: it’s the first CRISPR-based gene-editing therapy to be approved in the U.S. As importantly, CASGEVY is a first-in-class treatment that offers the potential of a one-time transformative therapy for eligible patients with sickle cell disease,” Reshma Kewalramani, M.D., CEO and President of Vertex, said in a statement.
Samarth Kulkarni, Ph.D., chairman and CEO of CRISPR Therapeutics, said his company “had a vision to translate CRISPR technology into multiple breakthrough therapies,” adding that “this U.S. approval of the first-ever medicine using CRISPR gene editing is breathtaking, and a truly humbling moment for me personally and for the whole organization.”
Stephan Grupp, M.D., Ph.D., section chief of the Cellular Therapy and Transplant Section and Director of the Kelly Center for Cancer Immunotherapy at the Children’s Hospital of Philadelphia, called the CASGEVY program “groundbreaking.”
“CASGEVY has the potential to be a transformative treatment for patients, and I look forward to continuing the work to ensure eligible patients can access this therapy across the country,” Grupp said in a statement.
Sickle cell disease is debilitating—and life-shortening
Sickle cell disease is a debilitating, progressive and life-shortening disease, Vertex and CRISPR Therapeutics noted, with SCD patients reporting health-related quality of life scores well below the general population. Lifetime health care costs in the U.S. of managing SCD for patients with recurrent VOCs is estimated between $4 and $6 million.
SCD is an inherited blood disorder that affects the red blood cells, which are essential for carrying oxygen to all organs and tissues of the body, the companies noted. SCD causes severe pain, organ damage and shortened life span due to misshapen or “sickled” red blood cells.
The disease’s clinical hallmark is VOCs, which are caused by blockages of blood vessels by sickled red blood cells and result in severe and debilitating pain that can happen anywhere in the body at any time, the companies said. In the U.S., the median age of death for patients living with SCD is around 45 years. An existing cure for the disease is a stem cell transplant from a matched donor—but this option is only available to a small fraction of patients living with SCD because of the lack of available donors, the companies said.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.