immunotherapy
Premature babies diagnosed with bronchopulmonary dysplasia (BPD) face the risk of rehospitalization, delayed brain development, and respiratory problems throughout childhood. No FDA-approved therapies are available for BPD—which is why the new FDA designation for AyuVis's drug candidate, AVR-48, could lead to game-changing impact.
Fort Worth’s AyuVis Gets FDA Fast‑Track Designation for Its Drug for Babies with a Chronic Lung Disease
by David Seeley | Aug 23, 2024
Premature babies diagnosed with bronchopulmonary dysplasia (BPD) face the risk of rehospitalization, delayed brain development, and respiratory problems throughout childhood. No FDA-approved therapies are available for BPD—which is why the new FDA designation for AyuVis's drug candidate, AVR-48, could lead to game-changing impact.
MORE►
by Quincy Preston |
Sep 18, 2023
The deal with Fresenius Kabi aims to bring innovative cell and gene treatments closer to patients by advancing Lupagen’s proprietary cell and gene therapy delivery system, the companies said. Their "to the bedside" approach could help open cutting-edge treatments to local hospitals and patients worldwide.
MORE►

by Lance Murray |
May 17, 2023
The grant from the National Heart, Lung, and Blood Institute will fund a first-in-human Phase 1 clinical trial of a new generation of immunotherapies. This is the fourth NIH grant AyuVis has received since its founding in 2014, doubling total non-dilutive funding to $4.2 million. Co-Founder and CEO Dr. Suchismita Acharya calls the move to a human trial "a huge milestone for AyuVis."
MORE►
by Lance Murray |
Mar 9, 2023
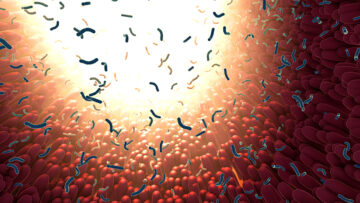
The researchers discovered how healthy bacteria can escape the intestine, travel to lymph nodes and cancerous tumors elsewhere in the body, and boost the effectiveness of certain immunotherapy drugs. “Now we understand that mechanism much better and, in the future, hope to use this knowledge to better fight cancer," said UT Southwestern's Andrew Y. Koh, M.D.
MORE►
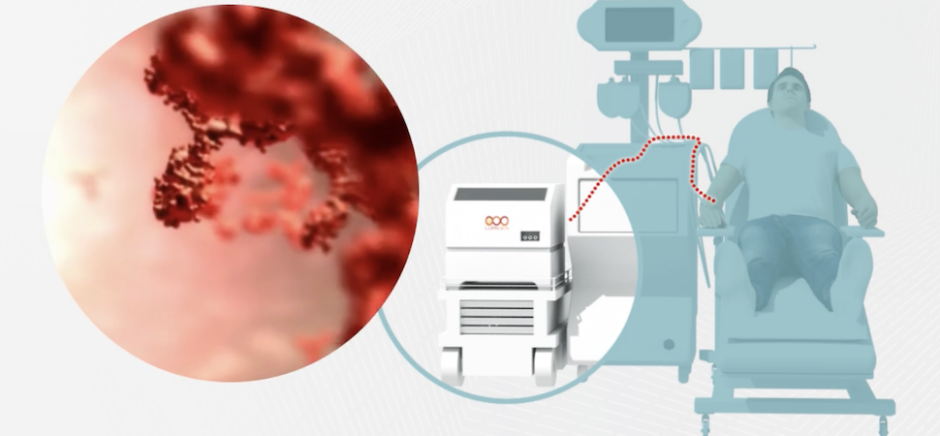
Dallas’ Lupagen Aims To Change the Way Cancer, Other Disorders Are Treated
by Kevin Cummings | Jun 2, 2022
Instead of shipping a patient's blood to a lab for customized cell therapy, Lupagen does the procedure "in vivo," using a dialysis-like treatment at the patient's bedside. Now it's collaborating with Umoja Biopharma to use its drug-delivery tech to place targeted immunotherapies in patients.
“We're able to deliver different types of gene therapies in a closed system," Lupagen's CEO says, calling the tech a "bridge to the future."
“We're able to deliver different types of gene therapies in a closed system," Lupagen's CEO says, calling the tech a "bridge to the future."
MORE►

by David Kirkpatrick |
Mar 31, 2022
Fort Worth-based AyuVis just received its second patent in three months for its immunotherapy treatments that protect pre-term babies from a debilitating chronic lung disease that affects many pre-term infants on ventilators.
The startup's lead drug candidate—AVR-48—is scheduled for clinical trials this summer.
The startup's lead drug candidate—AVR-48—is scheduled for clinical trials this summer.
MORE►