![]() Every week, we do a little research of our own. We’re looking for scientists, professors, engineers, entrepreneurs—anybody, really—engaging in research and development across North Texas.
Every week, we do a little research of our own. We’re looking for scientists, professors, engineers, entrepreneurs—anybody, really—engaging in research and development across North Texas.
There’s plenty of good work being done. If you want to put R&D under your microscope, sign up for our e-newsletter.
UTA researchers creating water modeling tool for Trinity River Authority
A team of researchers at The University of Texas at Arlington is using a $60,000 grant from the Trinity River Authority of Texas to develop a water resources modeling tool that the authority will use to plan water movement, storage, and use.

Nick Fang of the University of Texas at Arlington. [Photo: UTA]
Nick Fang, a UTA assistant professor in the Department of Civil Engineering, received the TRA grant for the project, and his team will create the water management and planning tool by using RiverWare, a modeling platform for operational decision-making and long-term planning for water reservoirs, rivers, lakes, and watersheds, UTA said in a release.
Fang’s team started the project in 2018 after receiving the same funding from TRA, with current research to continue developing the tool funded for this year.
The goal is aid in developing short- and long-term strategies in water management and planning for the increasing needs caused by the Dallas-Fort Worth region’s rapid growth, the release said.
“Our goal is to make this tool to assist the decision-makers in water authorities like the TRA so that the process will become less of a challenge than in years past,” Fang said.
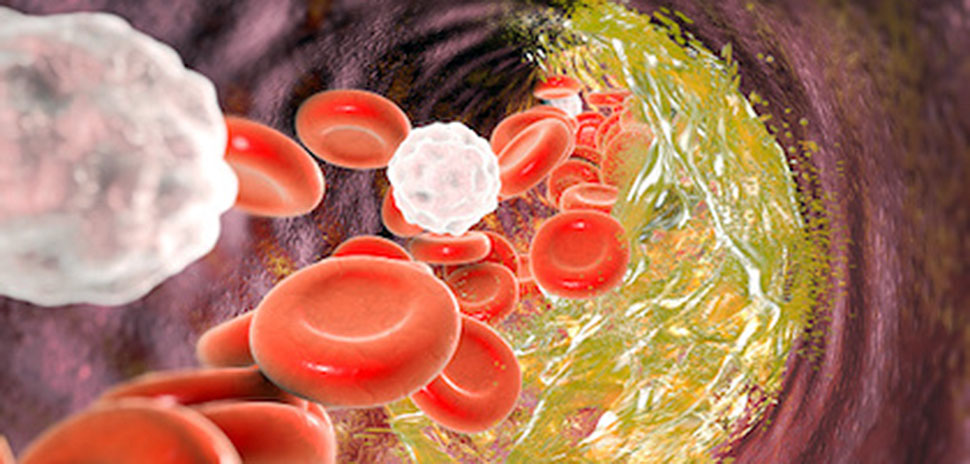
This Illustration depicts cholesterol causing a narrowing of an artery. [KATERYNA KON/SCIENCE PHOTO LIBRARY via Getty Images]
UTSW researchers make discovery about ‘bad cholesterol’
Many of us have been affected by the damage caused by the so-called “bad cholesterol,” either personally or a relative.

Philip Shaul
That type of cholesterol, called low-density lipoprotein, or LDL, enters the artery walls to cause the plaque that narrows blood vessels, often leading to strokes or heart attacks.
Now, UT Southwestern researchers have figured out how circulating LDL enters the artery walls, leading to the development of atherosclerosis, or hardening of the arteries.
This research could lead to future treatments to decrease the occurrence of these serious, often life-threatening conditions.
Since low-density lipoprotein, or LDL, cholesterol entry into the artery wall drives the development of atherosclerosis, or hardening of the arteries, and atherosclerosis leads to heart attacks and strokes, future treatments preventing the process may help decrease the occurrence of these life-threatening conditions, said Dr. Philip Shaul, senior author of the study that was published online in Nature.
“At the start of this work it was surprisingly unknown how LDL enters the artery wall to cause cardiovascular disease,” Shaul, director of the Center for Pulmonary and Vascular Biology at UT Southwestern, said in a release. “The paper’s findings solve that mystery and counter many scientists’ prior assumption that LDL simply enters through sites of damage or disruption in the single layer of endothelial cells that serves as the artery wall’s protective barrier.” Find out more about his important research here, in Carol Marie Cropper’s report.
FDA grants Breakthrough Device designation to new Caris cancer test
Caris Life Sciences, the Irving-based innovator in molecular science focused on precision medicine, announced that the U.S. Food and Drug Administration has granted Breakthrough Device designation for its MI Transcriptome companion diagnostic test.
The test is designed to detect gene fusions in solid tumors, and is intended to assist clinicians in identifying patients who might benefit from treatment with specific targeted therapies. The biotech company said in a release that it plans to submit the assay for Pre-Market Approval in late 2019.
Caris said the test is a next-generation sequencing-based in vitro diagnostic test that uses RNA isolated from formalin-fixed paraffin embedded tumor tissue to detect all classes of structural rearrangements, including fusions, deletions, inversions, and duplications. It also measures expression and splice variants in patients diagnosed with cancer, Caris aid.
“The FDA Breakthrough Device designation for the MI Transcriptome CDx assay is a significant step in advancing precision cancer care for individuals with specific genetic profiles who could benefit from targeted treatment options,” said W. Michael Korn, MD, Chief Medical Officer of Caris. “This also is an incredible milestone for Caris and the company’s efforts to advance molecular science and cancer care by employing cutting edge technology for the detection of highly actionable molecular alterations.”
Earlier this week, Caris announced that it had acquired Denver-based Pharmatech, Inc., a pioneer of the original Just-In-Time research system with the largest research-ready oncology network.No financial details of the acquisition were released.
READ NEXT
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.