Irving-based InspiroGene by McKesson, a dedicated business focused solely on supporting the commercialization of cell and gene therapies, has published its inaugural 2024 Cell and Gene Therapy Report: Advancing the Future of Medicine.
InspiroGene’s launch was announced two weeks ago to enable manufacturers, payers, and providers to navigate the complex CGT commercialization landscape, ensuring patients can access the life-changing treatments they need.
“With more than 30 FDA approved cell and gene therapies available in the U.S., the potential to deliver transformative care, or even cures, to patients with serious illnesses has never been greater,” Joe DePinto, head of Cell, Gene, and Advanced Therapies, McKesson, said in a statement. “Despite the compelling science, significant barriers prevent many patients from obtaining these life-changing therapies. Our 2024 report delves into these challenges and proposes possible solutions to broaden access for more patients.”
The report is designed to deliver unique insights about the cell and gene therapy (CGT) landscape and its future trajectory and includes an overview of the U.S. CGT pipeline, findings from a survey of 124 U.S. oncologists capturing their perspectives on these therapies, a newly developed interactive map of qualified CGT treatment centers nationwide, and interviews with industry experts, the company said.
[Graphic: InspiroGene]
Results of the survey on CGTs
The company said InspiroGene conducted a survey of 124 oncologists to better understand the current use of CGTs and possible barriers to future adoption. The goal of the survey was to gather insights on how, when, and why these specialists prescribe these therapies to their patients.
InspiroGene said the key findings from the report include:
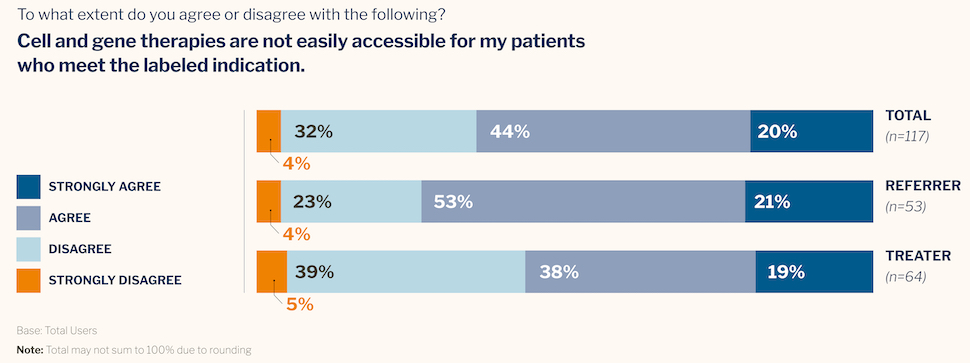
- 99% of oncologists agree that CGTs are among the most important medical innovations of our time. However, 64% also agree that therapies are not easily accessible for patients who meet the labeled indications.
- Three out of five physicians say patients they refer for CGTs often receive other treatments instead. When asked, they cite insurance coverage and out-of-pocket costs as the most common reasons.
- 47% of oncologists say their patients are “rarely” or “never” aware that CGTs are available for their condition.
The company said the report also explores challenges to equitable access to CGTs, including the concentration of delivery of care at academic medical centers in major metropolitan areas, leaving many Americans with little to no access. McKesson said its research into qualified treatment centers across the U.S., depicted in the report and in an interactive map, illustrates that “CGT deserts” exist across the nation.
According to McKesson, the report highlights the barriers to advanced care faced by patients and their providers in these underserved areas and it examines strategies for transitioning treatment into community settings.
Other features of the report include:
- Pipeline data illustrating development of CGT in broader therapeutic areas, beyond oncology, such as cardiovascular disease, diabetes, and central nervous system disorders.
- Expert perspectives by leaders from medical centers, research centers, community practices, and industry think tanks, exploring barriers to CGT adoption and potential solutions.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.