While chemists have always worked to develop solutions to people’s health problems, never have they been more in the spotlight in recent years than during the ongoing development of COVID-19 vaccines. These vaccines may have seemed to take a while to be created by the public eye, but were actually developed in record time.
Southern Methodist University researchers are looking to create more speedy results in the overall drug discovery process through ChemGen. The patent pending product is a set of computer-driven routines that can mimic chemical reactions in a lab, according to a statement.
Currently, it can take up to 15 years for a drug to make it completely through the design, development, testing, and approval process. And the process doesn’t come cheap—according to the Tufts Center for the Study of Drug Development, it takes over $2.6 billion for a manufacturer to get a new drug from the lab to the pharmacy.
That’s where ChemGen comes in.
“ChemGen has the ability to replace a team of 20 highly-skilled organic chemists in the optimization of a molecule of interest,” John Wise, the lead inventor of ChemGen and an SMU professor who specializes in structural biochemistry, said in a statement. “We’re basically arming an army of smaller labs to do really sophisticated research.”
The early part of the drug discovery process known as pharmacological optimization, which makes a drug functional and effective for specific applications, can be sped up by using ChemGen’s computational routines, according to a statement.
This process can take months for a team of chemists to complete, but it takes only a few days for ChemGen to do by using high performance computers like SMU’s ManeFrame II.
Through ChemGen, a team of chemists could potentially increase their productivity from working on as few as six problems a year to as many as 60, according to Wise. Smaller labs could use ChemGen to contribute to research at a level they cannot otherwise afford and major drug companies could benefit from the product, too.
“That’s going to make new drugs come out faster and cheaper, which is exactly what we need for the coronavirus and whatever comes next,” Wise said.
A time and money-saving process
“When a drug company finds a drug hit—a chemical ‘key’ that they think could be valuable—they might have a team of very skilled chemists work on that one targeted molecule. That’s not the only molecule they’ll work with, but they might spend three months of the next year making 1,000 variations of that one molecule,” Wise said.
The traditional approach to pharmacological optimization happens when chemists work to figure out if there’s a better match to a target protein than the one they just found, according to a statement.
The reason chemists do this is because if a drug doesn’t fit the protein perfectly, it won’t bond tightly enough with that protein to be effective, according to a statement. They also need to figure out if other proteins in the body might be blocked by that same chemical key, which could possibly result in unintentional side effects.
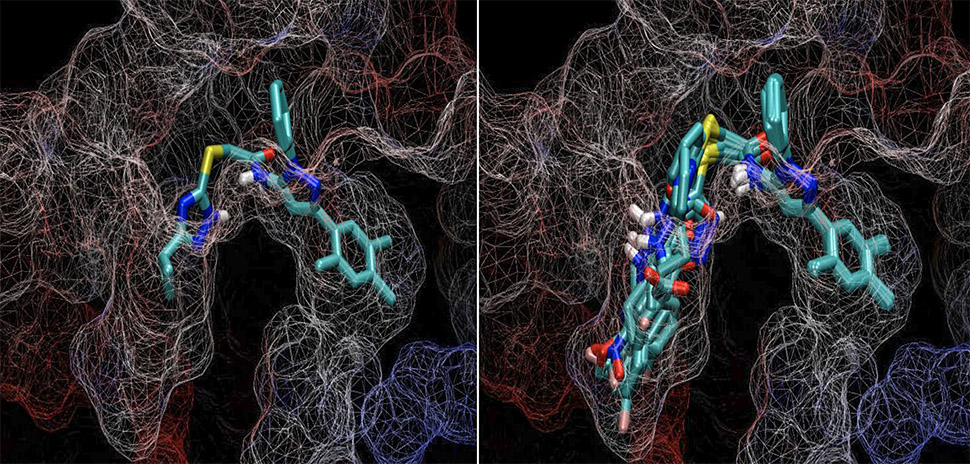
ChemGen is able to speed up this process by creating molecular variants of the original chemical key computationally instead of in a physical lab. It can copy what would happen under a various combinations of circumstances, according to a statement.
“We taught ChemGen the rules of chemistry for these reactions—what can be done and what can’t be done. We can take a thousand compounds, react them in the computer, and make 1,000 products from that. Then we can take that group of 1,000 and react them with a second group of 1,000 other molecules to create a million different, but related products,” Wise said. “This generates an enormous quantity of chemical variance for a given molecule.”
According to Wise, this leaves researchers with the molecules with the best chances of being improved, while thousands of unimproved molecules remain in the computer.
“This approach is very efficient in both time and money,” Wise said. “It limits waste and makes it more likely that the new drug will be better than what was originally discovered.”
The genesis of ChemGen
Wise originally got the idea for ChemGen while he was working with Pia Vogel, a professor and director of SMU’s Center for Drug Discovery, Design and Delivery, to try to find compounds that could reverse chemotherapy failure in aggressive cancers.
The resulting product has been over a decade in the making.
Alex Lippert, an associate professor in chemistry; Maha Aljowni, a PhD student of Lippert; Robert Kalescky, SMU’s HPC Applications Scientist; Ketetha Olengue, a former SMU student; and Amila K. Nanayakkara, Mike Chen, Maisa Correa de Oliveira, and Lauren Ammerman, SMU students in the Biological Sciences Ph.D. program, all contributed to the development of ChemGen.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.