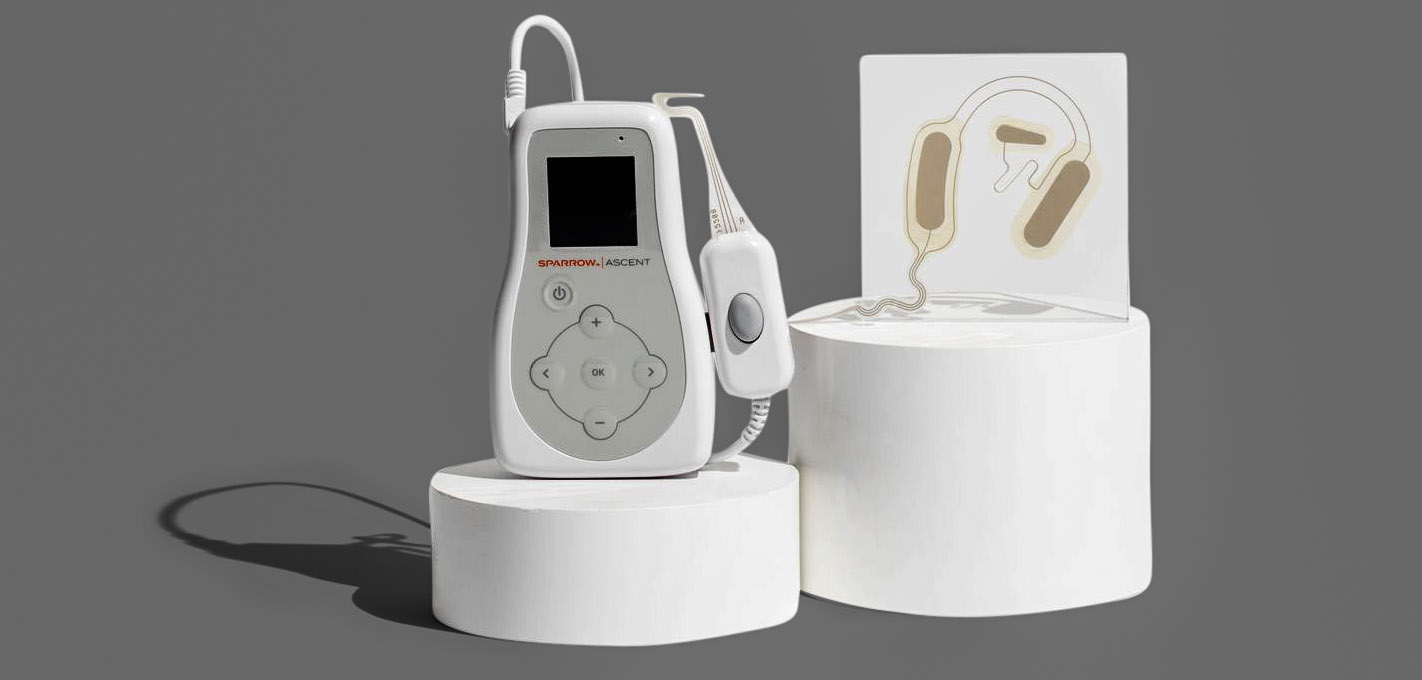
Spark Biomedical, a Dallas-based leader in wearable neurostimulation solutions, has launched telehealth services for its opioid withdrawal relief device Sparrow Ascent, which received FDA clearance in July.
Through Spark partnerships with existing telehealth networks, Sparrow Ascent can now be prescribed, and e-prescriptions administered, via remote patient access, the company said.
“We’re thrilled to introduce Sparrow Ascent to the vibrant telehealth services channel for patients, achieving a significant milestone in our ‘all-paths’ mission to provide accessible and patient-centric solutions,” Daniel Wagner, chief commercial officer of Spark Biomedical, said in a statement. “With the addition of telehealth, we’re removing the friction that patients may face when seeking treatment, ensuring the opportunity to receive the care they need, when and where they need it.”
Spark says Sparrow Ascent is a safe, drug-free opioid withdrawal device that provides a treatment option to patients seeking to decrease or eliminate their reliance on opioids and who can’t—or won’t—access facility-directed care.
Offers more comfortable and flexible home treatment

Spark Biomedical CEO Daniel Powell [Image: Spark Biomedical; DI background]
The company said that by pairing Sparrow Ascent and the telehealth home-based treatment option, a person’s road to recovery can be more comfortable, more flexible, and more accessible, empowering them to take that first critical step toward recovery.
Spark said that in 2020, roughly 40.3 million people experienced substance use issues, but only 4.8 million sought treatment. When patients facing opioid withdrawal do seek treatment, they often encounter obstacles when seeking treatment, including limited facilities and limited bed space to treat opioid withdrawal patients; a lack of comfortable, drug-free treatment options for those who prefer that recovery path; and a lack of therapy flexibility that aligns with the patient’s living situation and work requirements
To alleviate those challenges, Spark Biomedical said it has partnered with regional and national telehealth providers to introduce opioid withdrawal treatment access that eliminates barriers to care and provides safe, comfortable, drug-free opioid withdrawal therapy.
System was initially designed for in-patient settings
Spark said it has “reimagined and enhanced” its product to aid a broader range of patients, by building upon the success of its Sparrow Therapy System, which initially was designed for in-patient clinical settings.
The Sparrow Ascent was designed to be user-friendly, flexible, and safe enough to be utilized in various settings, including facility-directed, out-patient, or even in the comfort of patients’ homes through telehealth.
When paired with telehealth prescribing access, the company said these Sparrow Ascent advancements empower patients, granting them greater control over their opioid withdrawal therapy and opening the door to receive treatment on their own terms.
The company added that utilizing the Sparrow Ascent and a patient-centric model is only possible because the device provides physicians, patients and caregivers:
:: Safety, by offering a non-addictive, non-abusable, diversion-free treatment option. There are also no known drug interactions or systemic side effects;
:: Comfort, because unlike other opioid withdrawal treatment options, there is no need to experience withdrawal before starting therapy. Patients also maintain control of therapy strength throughout treatment— whether facility-directed, out-patient, at home or on the go; and
:: A drug-free solution. Sparrow Ascent uses mild electric pulses instead of pills, eliminating the need for X-waiver restrictions like induction management and daily clinic visits. As a drug-free option, Spark says it empowers the patient with control over their sobriety date and gives family and caregivers peace of mind.
“As we mark our five-year anniversary, we reflect on the profound impact of the opioid epidemic on individuals and communities,” Spark Biomedical CEO Daniel Powell said in a statement. “We remain steadfast in our mission to combat this crisis, The launch of telehealth paired with SparrowAscent will expand access to safe, comfortable, drug-free treatment for those struggling to get off opioids. This milestone represents our unwavering commitment to innovation, improving patient outcomes, and making a meaningful impact on the opioid epidemic.”
Patients will begin using the system with a simple quiz on SparrowRx.com. Once the quiz is done, patients are connected with a licensed telehealth provider who will assess suitability for the Sparrow Ascent treatment.
If deemed appropriate, that provider will issue an e-prescription that’s dispensed via a partner mail-order pharmacy, and Sparrow Ascent will arrive at the patient’s home within two business days, the company said.
Spark said that during the initial launch phase for telehealth, services are only available in Texas and California. More states will be added to both the telehealth and facility-directed offerings throughout the coming months, the company said.
Follows news of Spark subsidiary Five Liters’ “first in-human” studies
The Sparrow Ascent telehealth update follows follows a late-July announcement that Five Liters, a subsidiary of Spark Biomedical, is set to make its own strides in bioelectric wearable technology.
The new group is conducting “first in-human” studies to demonstrate the effectiveness of noninvasive wearable neurostimulation in reducing blood loss, particularly for individuals with blood disorders or undergoing surgical procedures. You can read more about that in our story here.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.