Dallas’ Southern Methodist University researchers have written and developed a computational approach to drug discovery that’s meant to expedite the synthesis of derivative molecules normally made in a lab by mimicking the chemical reactions through computer-driven routines.
Currently, the process of research, development and approval of a new drug can take up to 12 to 15 years, and it costs more than $2.6 billion for a manufacturer to get it from the laboratory to a pharmacy’s shelf, according to the Tufts Center for the Study of Drug Development.
However, SMU’s development, called ChemGen intends to cut the time and costs associated with drug discovery, specifically to enable smaller labs to contribute more research.
John Wise, lead inventor of ChemGen and an SMU associate professor of biological sciences, said the approach could potentially empower a building full of skilled chemists to dramatically increase their productivity from working on as few as six problems a year to as many as 60.
“This approach is very efficient in both time and money,” he said. “It limits waste and makes it more likely that the new drug will be better than what was originally discovered.”
ChemGen, which is patent pending, works by speeding up the pharmaceutical optimization, a process that normally takes scientists months to accomplish. With the help of ChemGen and high performance computers, early drug discovery could be fulfilled in a few days.
The first step to creating a new drug is identifying a molecular target it can act on, Wise said. This target plays a role in why a person infected by a virus will feel symptoms.
Once identified, scientists search for chemical keys that can potentially block the target’s function and prevent the negative biological effects of a disease.
“When a drug company finds a drug hit—a chemical ‘key’ that they think could be valuable—they might have a team of very skilled chemists work on that one targeted molecule,” Wise said. “That’s not the only molecule they’ll work with, but they might spend three months of the next year making 1,000 variations of that one molecule.”
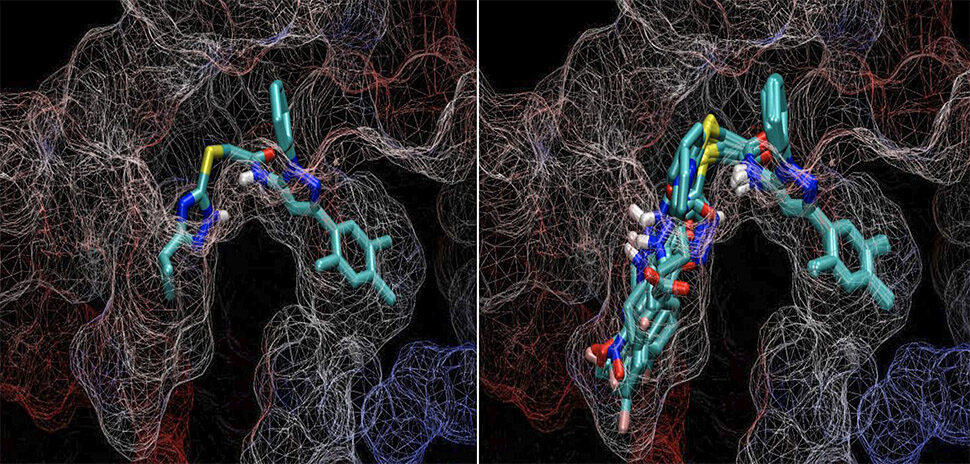
Unlike the traditional approach to pharmaceutical optimization that tests in a physical labratory, ChemGen creates molecular variants of the chemical key computationally.
This allows ChemGen to look at those variants and determine which is the best match for the targeted protein than the original key. “We taught ChemGen the rules of chemistry for these reactions—what can be done and what can’t be done,” Wise said.
ChemGen can be potentially applied to pharmaceutical, computational medicine, drug discovery, drug development, and chemical synthesis.
Wise has been working on ChemGen with the collaboration of other SMU scientists and students for more than a decade. His original idea was inspired by his work to find compounds that can reverse chemotherapy failure in aggressive cancers with Pia Vogel, SMU professor and director of the Center for Drug Discovery, Design, and Delivery.
“I would also hope that major drug companies take advantage of this technology, too,” Wise said. “That’s going to make new drugs come out faster and cheaper, which is exactly what we need for the coronavirus and whatever comes next.”
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.