An SMU chemist and her colleagues are investigating what happens in the tiny world in which atoms exist and interact with their work to develop a computational tool to provide those answers.
“Understanding interactions at the atomic level could aid us in caring for our planet, impact the ways drugs interact with the human body, and even let us explore organic materials that have come to Earth thanks to comets and asteroids or simulate chemical reactions taking place far out in space including star dusts or interstellar grains,” chemist Elfi Kraka said in a statement.
SMU said mathematical functions used to calculate the potential energy of a system of atoms are known as interatomic potentials.
It said that machine learning interatomic potentials (MLIP)s have become an efficient and less expensive alternative to traditional quantum chemical simulations, which even on today’s high-performance computing often become out of reach for larger systems.
Graphic: Nature Chemistry
ANI-1xnr: what it is and how it was tested
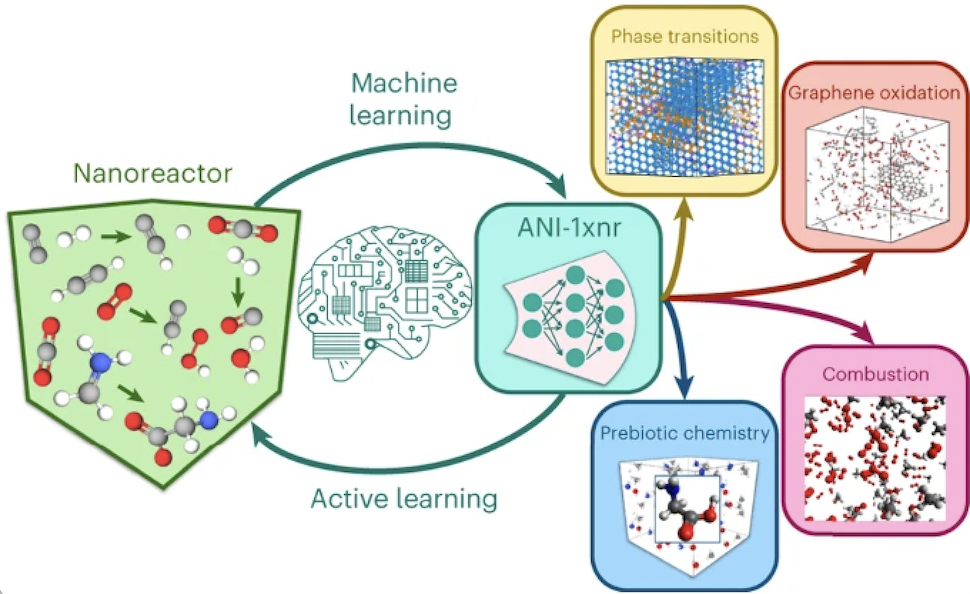
The researchers developed a novel MLIP called ANI-1xnr that is applicable to a broad range of reactive chemistry without costly refitting, a major drawback of the MLIPs currently in use, SMU said.
The researchers’ findings appear in the journal Nature Chemistry.
SMU said that Kraka and her colleagues put their new ANI-1xnr potential to the test, simulating five distinct systems in extreme environments: carbon solid-phase nucleation, graphene ring formation from acetylene, biofuel additives, combustion of methane, and the spontaneous formation of glycine from early earth small molecules (the famous Miller experiment), with all five closely matching available experimental or theoretical data.
According to SMU, Miller’s experiment was conducted in 1959, applying electricity to a mixture of simple molecules, such as ammonia, carbon monoxide, water, hydrogen, and methane uncovering how amino acids — important life-building blocks—were formed. Miller’s work led to the creation of a new field of study called prebiotic chemistry, which aims to understand the chemical processes and reactions that occurred on Earth before life emerged.
Since then, SMU said that scientists have referred to Miller’s experiment to understand specific reactions that lead to the formation of amino acids under Early Earth conditions.
The ANI-1xnr dataset is now available publicly for research use, SMU said.
SMU said the study’s authors intend expand and further train ANI-1xnr over time, where Kraka is interested in exploring the formation of amino acids and precursors of DNA from small molecules under extraterrestrial conditions, one of her recent research interests.
CATCO research and partners
Kraka is head of the Computational and Theoretical Chemistry Group (CATCO) at SMU.
SMU said that CATCO’s research mission is to develop modern quantum chemical tools and to apply them to solve pending problems in chemistry, biology, materials science, astrophysics, and beyond.
CATCO is one of the high-volume user groups of SMU’s high performance computing cluster, SMU said. Malgorzata Makos, another paper author, is a CATCO graduate who interned at the Los Alamos National Laboratory and now works Oak Ridge National Laboratory in Tennessee, SMU said.
Additional authors include: Shuhao Zhang, Carnegie Mellon University and Los Alamos National Laboratory; Ryan Jadrich, Kipton Barros, Benjamin T. Nebgen, Sergei Tretiak, Nicholas Lubbers and Richard Messerly from Los Alamos National Laboratory; Olexandr Isayev from Carnegie Mellon University; and Justin Smith from Los Alamos National Laboratory and NVIDIA Corp.
Funding for the study’s CATCO component was provided by a National Science Foundation grant.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.