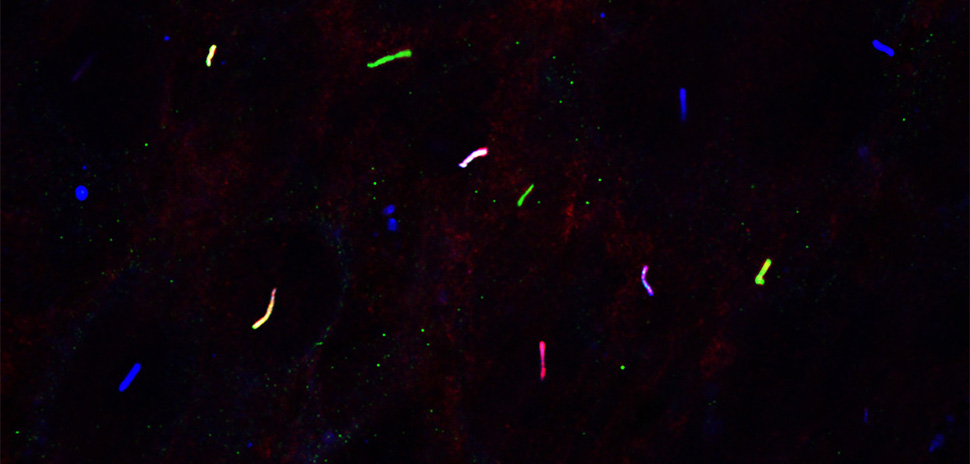
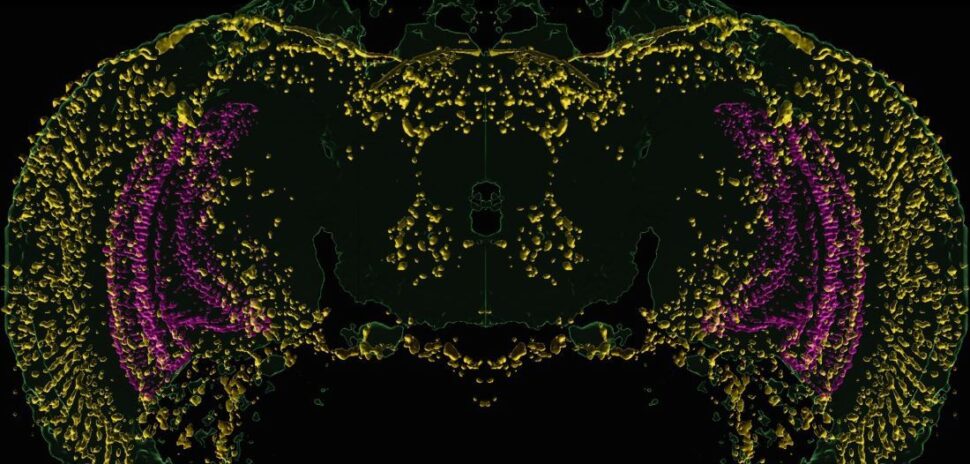
The weight-loss drug market has surged in recent years, but most treatments still work through the same biological pathway. UT Southwestern researchers have identified a gene, Gpr45, that controls appetite through a different mechanism.
The finding, recently published in Science, could open the door to obesity drugs that work alongside existing treatments.
When Gpr45 doesn’t function properly in mice, the animals overeat and become obese. The gene acts as a transporter in brain cells, helping move proteins to the right place to regulate appetite. Disabling the gene caused young mice to begin overeating by six weeks of age.
Study leader Zhao Zhang suggested that developing drugs to increase GPR45’s activity could offer a novel approach to fighting obesity, which affects about 40% of U.S. adults.
“This research uncovers a previously unknown signaling pathway in tiny, antenna-like structures on brain neurons that plays a critical role in controlling appetite, opening new doors for anti-obesity treatments,” said Zhang, assistant professor in the Center for the Genetics of Host Defense and of Internal Medicine at UT Southwestern, in a statement.
Gene linked to appetite control
The UT Southwestern team used a tool developed in-house called Automated Meiotic Mapping, or AMM, to pinpoint the gene’s role. Originally created by UTSW Nobel Laureate Bruce Beutler, M.D., the system uses induced mutations in mice and AI-driven analysis to rapidly trace physical traits back to specific genes—often in real time.
Using AMM, the researchers identified two mutations in Gpr45 that caused mice to gain weight rapidly, even on a standard diet. When the team deleted the gene using CRISPR, the result was the same: significant overeating and obesity.

Zhao Zhang, Ph.D. [Photo: UTSW]
That’s timely as GLP-1-based drugs like Ozempic and Wegovy have reshaped the obesity treatment market.
“The influx of weight-loss drugs in recent years has revolutionized the health care industry, not only delivering sustained results but also providing significant benefits to cardiovascular health, blood sugar management, and regulation of blood pressure and cholesterol,” UTSW said.
The researchers found that GPR45, the protein encoded by the Gpr45 gene, is active in neurons in the hypothalamus, the brain region that regulates hunger. It’s located in primary cilia, the small antenna-like structures on brain cells that help transmit signals.
Other appetite-linked proteins, such as those from the well-known MC4R gene, are also found in these cilia. But until now, their function there had remained unclear.
Zhang’s team discovered that GPR45 acts as a transporter, moving a key signaling protein called Gαs into the cilia. There, it helps activate MC4R to suppress appetite. Without GPR45, MC4R remains inactive, disrupting appetite control and leading to overeating.
While two FDA-approved drugs already target MC4R, they’re only approved for rare genetic conditions and have limited commercial use. Zhang said developing drugs that boost GPR45 activity could offer a new way to regulate appetite more broadly.
The team behind the discovery
Other UTSW researchers involved in the study include co-first authors Yu Xun, Ph.D., and Yiao Jiang, Ph.D., as well as Saikat Mukhopadhyay, M.D., Ph.D.; Chen Liu, Ph.D.; Sara Ludwig, Ph.D.; Miao Tang, M.D.; and Baijie Xu, Ph.D. Bruce Beutler, M.D., who co-developed AMM, also contributed to the study.
Beutler holds the Raymond and Ellen Willie Distinguished Chair in Cancer Research, in Honor of Laverne and Raymond Willie, Sr. He is a Regental Professor and member of the Harold C. Simmons Comprehensive Cancer Center.
The study was funded by grants from the National Institutes of Health and Japanese research agencies including JST Moonshot R&D and AMED-CREST.
Earlier this year, we told you about how a study co-led by Beutler may have helped researchers open the door to therapies that could target a little-known protein that helps blood cancers stay alive and potentially shut it down—without harming healthy cells.
Maddie Preston contributed to this report.
Don’t miss what’s next. Subscribe to Dallas Innovates.
Track Dallas-Fort Worth’s business and innovation landscape with our curated news in your inbox Tuesday-Thursday.