Lupagen is teaming up with a Seattle-based preclinical biopharmaceutical startup to change the way cancer is treated.
The Dallas-based medical device and gene therapy company has entered into a new collaboration with Umoja Biopharma to use its therapeutic delivery technology to place targeted, cell-changing immunotherapies inside a patient at the bedside.
“We have a bridge to the future of in vivo therapies, because we offer an alternative delivery modality,” Lupagen CEO Nipon Das told Dallas Innovates. “We can allow a situation where you can use a lower-dose product, because we concentrate the target cells in one place.”
Tackling cost and complexity

Nipon Das, CEO of Lupagen. [Photo: Lupagen]
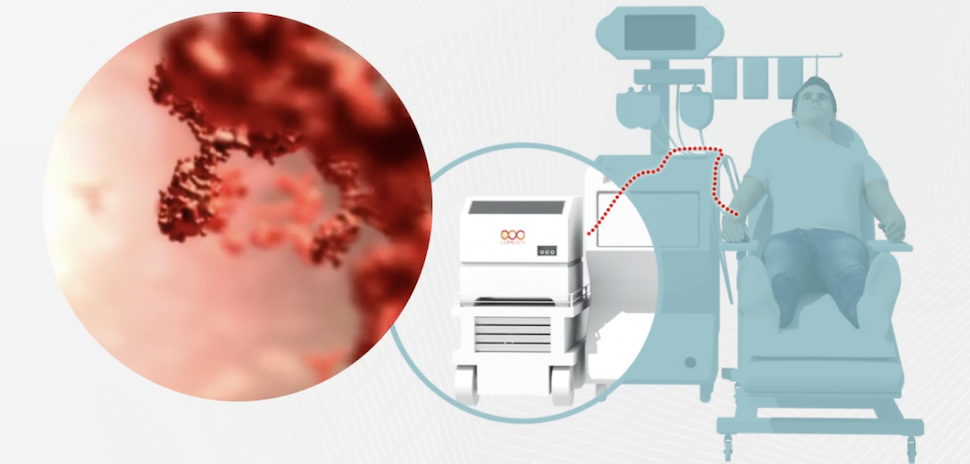
As part of the move, Lupagen will use its drug delivery system, which removes blood from a patient’s body, separating it into subsets and combining it with Umoja’s immune cell reprogramming therapeutics that target solid tumors and cancers that start in blood-forming tissues. Das likens the process to a dialysis treatment.
“We’re able to deliver different types of gene therapies in a closed system. All of the targeting and all the binding happens in our system, then it gets transported back to the patient,” Das said. “There’s a wide range of different types of indications we can go after.”
Das said that by using in vivo treatments, as opposed to ex vivo—where blood is shipped to a specialized lab for making customized cell therapy before being replaced in a patient’s body—the cost of using Lupagen’s system is cheaper. By using a patient’s own blood cells, the likeliness of the body rejecting the therapy is reduced. And by being specifically targeted to an individual patient, less drugs are needed, resulting in fewer negative side effects, Das added.
“The biggest challenges that the cell and gene therapy community is facing are cost and complexity of production,” Das said.
Collaborative growth
While testing the efficacy of the delivery system for Umoja’s therapy, Lupagen has agreed to not develop or commercialize its device for the delivery of viral vectors in the oncology field. In turn, Umoja retains the right to an exclusive global deal to develop Lupagen’s device in the field of oncology.
Das said that through other development contracts and collaborations like the one with Umoja, Lupagen has been able to fund itself without taking on an institutional money. Umoja has gone a different route, raising a $210 million Series B funding round co-led by SoftBank Vision Fund 2 and Cormorant Asset Management last June, which helped it break ground on a new 146,000-square-foot development and manufacturing facility in Colorado.
“We’ve been benefiting from the fact that the industry has also been learning,” Das said. “There’s this explosion of different types of in vivo therapy, and I think as developers realize that there are certain challenges or certain areas they’d like to optimize, we fill a small niche of being able to complement some of their clinical programs.”
Lupagen has its own therapeutics program
While Lupagen is working with Umoja on controlled viral vector targeting of T cells, Das said Lupagen’s device is able to take any type of peripheral blood mononuclear cells and target those for a number of other things like other autoimmune and protein deficiency disorders. In addition, the company has its own internal therapeutic program, using lipid nanoparticles, polymeric nanoparticles, and other non-viral delivery systems.
Lupagen was founded in 2017 and has grown to a team of about twelve. Das said a small team runs the company’s business operations from a space in the Cypress Waters development in Coppell, with its biological work taking place remotely and its hardware engineering team working in the Chicago area.
Looking ahead, Das said the company plans to build out its biology team, likely in the Dallas or Chicago area, depending on where Lupagen is able to source new talent.
“The potential for the cell and gene therapies to address a wider group of patients, a wider range of indications, is really today constrained by a lot of the cost of production and development,” Das said. “If we can bring the cost of goods down to be at parity with current small molecules, biologics, or monoclonal antibodies, it opens up the aperture to treat a wider group of patients, a wider group of indications, versus just being reserved for very niche and rare.”
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.