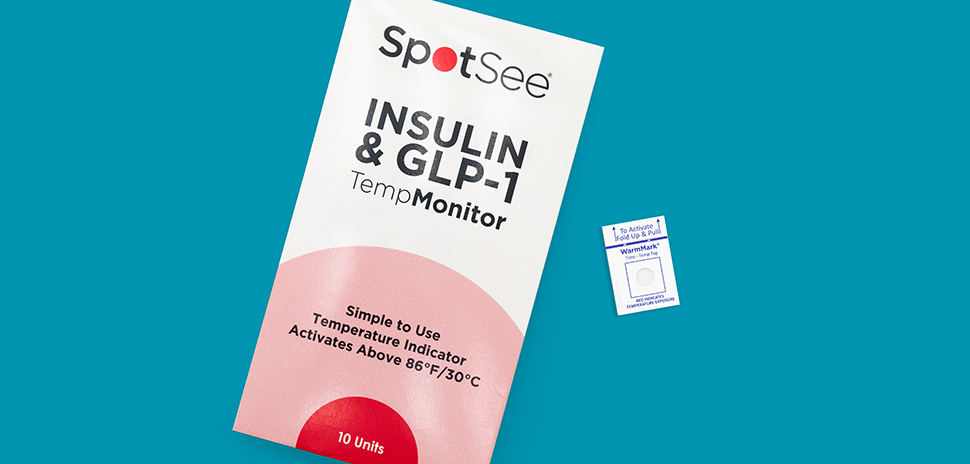
Dallas-based medtech company SpotSee has launched TempMonitor, a simple and affordable single-use temperature indicator designed for patients who rely on insulin and Glucagon-like, peptide-1 (GLP-1) medications.
“At SpotSee we’re focused on building solutions that span from pallet to patient and SpotSee’s TempMonitor addresses the critical need for monitoring the storage of patient’s temperature-sensitive medication, including Insulin and GLP-1s,” President and CEO Tony Fonk said in a statement. “We recognize the challenges faced by patients and their families who depend on these types of medications every day, all around the world. By using TempMonitor, patients can gain peace of mind knowing that their medications are stored and transported within the recommended temperature range.”
SpotSee’s TempMonitor product will be on display at LogiPharma 2024, April 16 to 18 at Centre de Congrès de Lyon in Lyon, France.
Utilizing Spotsee’s WarmMark tech
SpotSee said that TempMonitor uses its WarmMark technology to provide visual evidence if a medication is exposed to unacceptable temperature levels. The mini single-use indicators have a simple color-changing mechanism that alerts users when the temperature exceeds 30 degrees Celsius (86 degrees Fahrenheit), signaling possible compromise of product quality.
Discreet and easy to carry, TempMonitors are a convenient solution for users, the company said. The monitors are packaged in units of 10 and are available for purchase in the U.S. and Canada.
SpotSee said that the window of the TempMonitor indicator will stay white as long as no evidence of temperature excursions exist.
Should the temperature climb beyond 30 degrees Celsius (86 degrees Fahrenheit) within 30 minutes, the indicator will show red, which indicates that the product was exposed to higher than recommended conditions. Patients then know how long the product had been exposed above the threshold temperature.
At the LogiPharma conference in France, SpotSee CEO Fonk will moderate a panel focused on the transformation of digital capabilities to improve visibility, quality, and reactivity in the temperature-controlled supply chain.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.