Dallas-based Articulate Labs has been accepted into the Medical Device Innovation Consortium’s (MDIC) Advanced Manufacturing Clearing House (AMCH) initiative.
The Arlington, Virginia-based MDIC is piloting the AMCH program, with support from the U.S. Food and Drug Administration, to help encourage the medical device industry to adopt advanced technology not just during device production but across the total product life cycle, Articulate Labs said. The goals for implementing advanced manufacturing technologies include improving product quality, increasing operational effectiveness, and increasing product intelligence.
First in AMCH program to offer digital twin technology
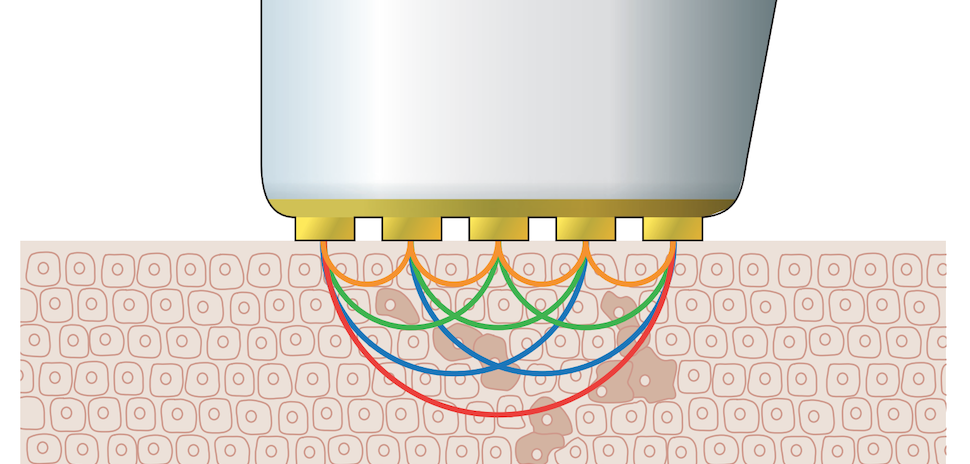
Articulate Labs, which is based out of Biolabs Pegasus Park, said the real-time operating system (RTOS) enabling its devices integrates a variety of metrics collected from patients within a digital twin—a digital model of a real-world physical system or product—to better personalize therapeutic capability and to potentially offer diagnostic feedback to providers.
The company said its first, publicly visible application for this technology is in gait-synchronous electrical muscle stimulation of atrophied quadriceps (thigh) musculature toward accelerated knee rehabilitation. It said, however, that the underlying RTOS can be adapted, beyond addressing other joints or musculoskeletal conditions, to create more medical devices that take in a variety of environmental inputs and output high-resolution detail on physical systems and adaptive, semi-autonomous therapies.
Articulate Labs said that for other medical devices, RTOS construction also could enable use of lower cost processors, eliminate reliance on internet connectivity for real-time data processing, and offer internal error management, lowering the risk to patients in case of device failure.
The company said that over the course of six months, it will collaborate with MDIC to build a use case for Articulate Labs’ RTOS—how the technology was developed, how it was implemented, what lessons were learned in the process, and what measurable changes occurred after technology implementation. That use case then will be shared with the medical device community. Additionally, the AMCH program enables proactive interaction with the FDA throughout the project’s execution. Articulate Labs said it is the first company to be accepted into the AMCH initiative offering digital twin technology.
Device aimed at speeding recovery
In November, Articulate Labs was awarded $1.3 million by the Orthopaedic Research and Education Foundation, the Medical Technology Enterprise Consortium, and the U.S. Army’s Medical Research and Development Command.
“This award will fund a study using our novel wearable devices to accelerate return to duty for West Point Cadets recovering from ACL reconstruction,” Articulate Labs CEO and Co-Founder Josh Rabinowitz said in an email to Dallas Innovates.
The MDIC is the first public-private partnership that brings together representatives of the FDA, National Institutes of Health, Center for Medicare and Medicaid Services, NIST, and other agencies, industry, nonprofits, and patient advocacy organizations to improve the processes for development, assessment, and review of new medical technologies. It coordinates the development of methods, tools, and resources used to manage the total product life cycle of a medical device to improve patient access to cutting-edge medical technology.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.