Steve Rybka
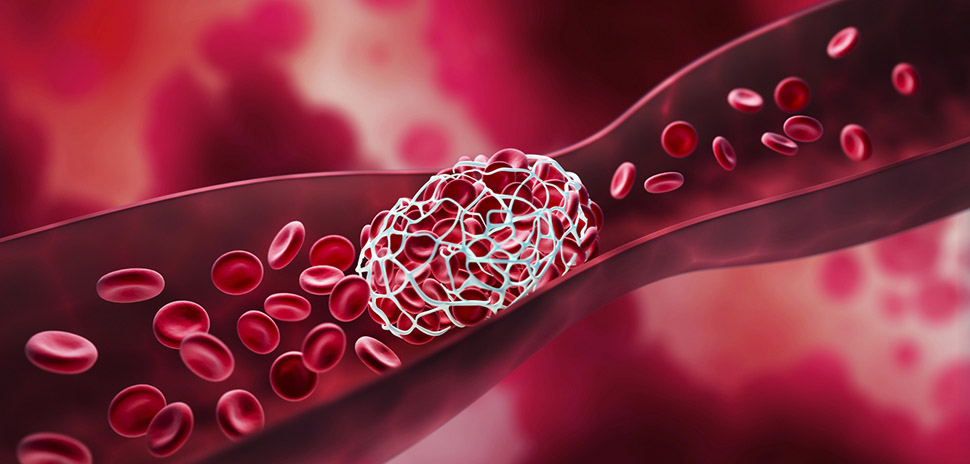
Vesalio—a privately held medical device company focused on advancing patient care in vascular diseases—has announced the U.S. commercialization of its pVasc Thrombectomy System. The mechanical device is designed for the non-surgical removal of peripheral occlusions, which are blockages in the arteries of the arms and legs caused by blood clots, inflammation, and narrowing blood vessels.
The mechanical system—a self-expanding nitinol structure on a pusher wire—provides “a versatile tool with no need for capital equipment,” the company said.
pVasc targets the full range of embolisms “from soft, acute clots to fibrin-rich, calcified ones, enabling a fast and safe removal,” Vesalio added, noting that its Drop Zone technology traps, retains, and securely removes the blood clot to restore flow in patients suffering from acute limb ischemia (ALI) and other conditions related to peripheral artery disease, or PAD.
“PAD is a common disease that can lead to devastating consequences,” Vesalio CEO Steve Rybka said in a statement. “With the pVasc Thrombectomy System, physicians now have access to a critical tool to help their patients. We received extremely encouraging early feedback on pVasc’s ease of use and efficacy in cases with challenging clots. At Vesalio, we remain steadfast in our commitment to provide innovative technologies to physicians treating vascular occlusions.”
Vesalio moved HQ to Plano and hired a new CFO in May

J.D. McCulloch
In May, Vesalio announced its headquarters relocation from Nashville, Tennessee, to Plano, north of Dallas. The company—which was founded in 2013—saw the Dallas area as a growing hub for Fortune 500 companies, top private companies, and clusters of medical device companies. It said its new home was chosen for its “business-friendly environment, highly educated and skilled labor force, low cost of doing business, and world-class transportation infrastructure.”
That same month, Vesalio announced the hiring of J.D. McCulloch as its new CFO. McCulloch had most recently served as CFO and head of business development at Dallas-based Dialectic Therapeutics, an oncology-focused biotech company headquartered in Old Parkland.
One physician familiar with the use of pVasc is Dr. Frank Arko, chief of vascular and endovascular surgery and co-director of the Aortic Institute at Carolinas Medical Center, Sanger Heart and Vascular Institute, in Charlotte, North Carolina.
“Given the complicated health history of patients with ALI, physicians often face the challenge of delivering devices through highly diseased arteries and working in small, stenotic vessels,” Arko said in a statement. “pVasc has a low profile and excellent deliverability, making it an especially valuable tool when access is difficult or when working in arteries below the knee.”
Up to 12M U.S. adults struggle with PAD
PAD affects 10 to 12 million adults in the U.S. aged 40 and older, Vesalio noted, increasing their risk of amputation, heart attack, stroke, and death. Each year, around 185,000 amputations are performed in the U.S., with more than 2 million people currently living with an amputation. According to the American Heart Association, nearly half of people 65 and older who underwent a limb amputation due to PAD died within a year of surgery, the company added.
Dr. Nick Abedi, a vascular surgeon at Fayette Surgical Associates in Lexington, Kentucky, said that he has achieved “highly positive results with pVasc in challenging cases that range from organized emboli in the tibials to emboli in the brachial and distal ulnar artery.”
“pVasc has helped my patients avoid open surgery on multiple occasions, becoming an invaluable tool in my practice,” he added in a statement.
Vesalio said pVasc is indicated for arteries from 2 to 6 millimeters in diameter, typically located below the knee, in the femoral-popliteal, the mesenteric, the upper extremities, and a few other locations.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.