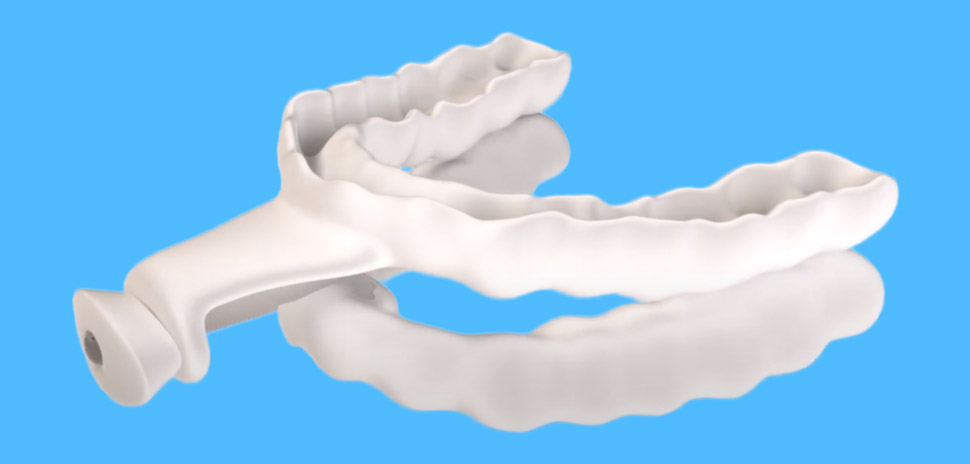
Farmers Branch-based Airway Management has launched its FDA-cleared Nylon flexTAP, calling it “the world’s first digitally printed single-point midline oral appliance device for sleep apnea.”
Cleared by the FDA for mild to moderate obstructive sleep apnea (OSA), the 3D-printed oral device combines the company’s “cutting-edge” patented Vertex Technology with “unparalleled comfort and efficacy.”
Worn during sleep, the device enables vertical and horizontal movement to maximize airway space, reducing the need for excessive protrusion. The company says that’s ideal for patients with larger tongues or narrow airways.
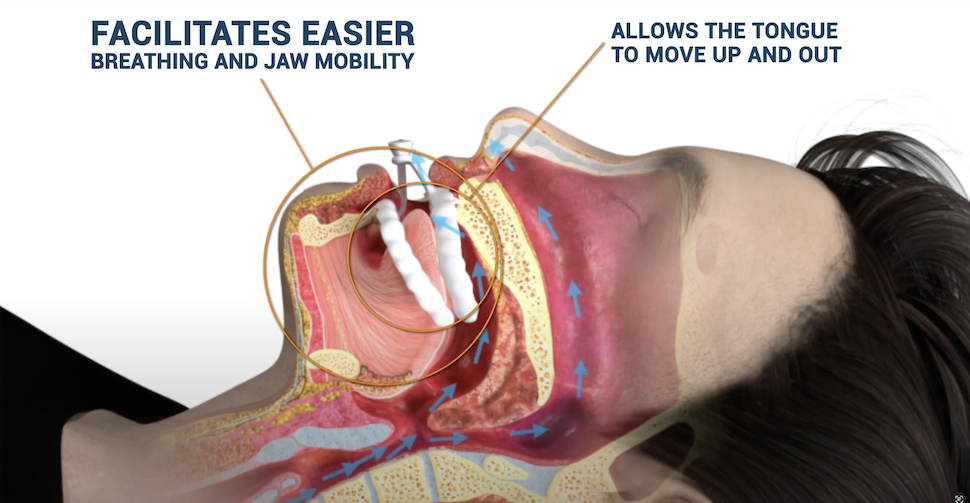
Airway Management’s Nylon FlexTAP device [Video still: Airway]
The wearers teeth rest within the device’s Ultra-Thin Custom TAP Trays, which Airway says offer maximum comfort without compromising treatment effectiveness. Bite registration isn’t required, which Airway says simplifies the fitting process, saving time for clinicians and patients alike.
BPA-free and metal-free, the device is crafted with biocompatible materials for enhanced safety, the company said.
Airway said the Nylon flexTAP includes a mouth shield and AM Aligner, making it the only oral appliance to include a nasal breathing accessory improving efficacy. The AM Aligner is a morning exercise tool which the company says mitigates common side effects associated with oral appliance therapy.
“We’re thrilled to introduce the Nylon flexTAP, a game-changer in oral appliance therapy,” Airway CEO Charles Collins said in a statement. “Feedback from key opinion leaders in our TAP Sleep Care system highlights its superior effectiveness and patient comfort. Peer-reviewed studies also confirm that our patented mouth shield, which promotes nasal breathing, significantly improves treatment outcomes. This innovation underscores our commitment to advancing sleep health.”
Since 2015, the American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine have recommended oral appliance therapy for mild to moderate sleep apnea. Airway Management says its new Nylon flexTAP sets a new benchmark by leveraging digital printing technology to create the first FDA-approved single-point midline device, eliminating the need for bite registration while maintaining precision.
You can see a YouTube video that further explains the device by going here.
Don’t miss what’s next. Subscribe to Dallas Innovates.
Track Dallas-Fort Worth’s business and innovation landscape with our curated news in your inbox Tuesday-Thursday.